Abstract
Normal erythroid maturation is highly regulated to maximize the production of HbA heterotetramer (α2β2) and minimize the accumulation of potentially toxic free α- or β-globin subunits. In β-thalassemia, β-globin gene (HBB) mutations cause buildup of free α-globin, which forms intracellular precipitates that impair erythroid cell maturation and viability. Endogenous mechanisms exist to detoxify free α-globin, as evidenced by clinical observations that most individuals with HBB haploinsufficiency (β-thalassemia trait) are asymptomatic. Previously, we showed that free α-globin is eliminated in erythroid precursors by the ubiquitin-proteasome (UPS) system and by a lysosomal-dependent process presumed to be autophagy (Khandros et al., Blood 2012;119:5265). Our current study investigates the latter.
Atg5 is required for basal autophagy and autophagosome formation via conjugation with Atg12 in most cell types. We investigated whether Atg5 played a selective role in eliminating free α-globin during unbalanced globin chain synthesis. β-thalassemic mice (HbbTh3/+) carrying a conditional Atg5 allele (Atg5fl) in which exon 3 is flanked by loxP sites were interbred with mice expressing erythroid-specific Cre recombinase (EpoR-Cre). Loss of Atg5 in β-thalassemic erythroid precursors inhibited autophagy, as evidenced by the accumulation of the markers p62/SQSTM1 and LC3. However, there was minimal effect on the β-thalassemic phenotype as measured by RBC count, Hb, hematocrit, reticulocyte count, and spleen weight. Moreover, loss of Atg5 did not alter the level of insoluble α-globin in β-thalassemic RBC fractions, as assessed by triton-acetic acid-urea (TAU) gel electrophoresis to resolve the α- and β-globin chains. Thus, Atg5, a key component of canonical autophagy, is not required for the elimination of free α-globin in β-thalassemia.
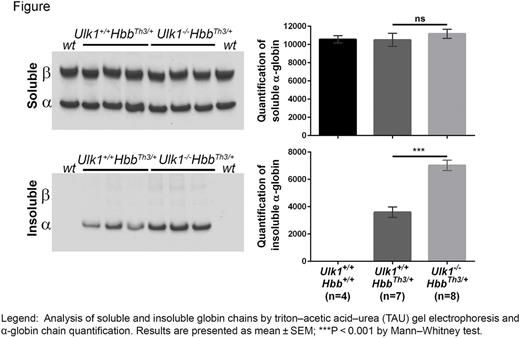
The Ulk1 (unc-51−like kinase 1) protein kinase participates in canonical and alternative autophagy pathways and mediates mitophagy during erythropoiesis. To determine the role of Ulk1 in a-globin degradation, we introduced a null allele into HbbTh3/+ mice. Ulk1−/− HbbTh3/+ mice were born at normal Mendelian ratio, but exhibited a high rate of perinatal death. Only 20% of double-mutant mice survived for 30 days (n=5), compared to 70% of Ulk1+/+ HbbTh3/+ mice (n=13) (P <0.05). To examine the consequences of Ulk1 loss in β-thalassemic erythroblasts, we transplanted whole bone marrow cells from double mutants and controls (CD45.2) into lethally irradiated wild-type hosts (CD45.1; >87% engraftment after 30 days). Loss of Ulk1 exacerbated the β-thalassemic phenotype, as evidence by reduced Hb (10.85 ± 0.18 vs 10.21 ± 0.18 g/dL, n=14 vs 8; P <0.01), reduced RBC number (7.37 ± 0.13 vs 6.35 ± 0.09 × 106/mL; P <0.0001), increased reticulocyte count (17.99 ± 2.76 vs 39.41 ± 2.14%; P <0.0001), and increased spleen weight (0.24 ± 0.01 vs 0.34 ± 0.03 g; P <0.01). Moreover, insoluble α-globin was increased by approximately 2-fold in Ulk1−/− HbbTh3/+ reticulocytes, ascompared to Ulk1+/+ HbbTh3/+ cells (Figure). To assess the turnover of newly synthesized free α-globin, we pulse-labeled reticulocytes with 35S amino acids and chased with or without a proteasome inhibitor (MG132) or a lysosome inhibitor (chloroquine or bafilomycin A1). Analysis of control (Ulk1+/+ HbbTh3/+) reticulocytesshowed that newly synthesized insoluble free α-globin was cleared by separate proteasomal and lysosomal pathways. In marked contrast, the lysosomal-dependent α-globin degradation pathway was completely eliminated in Ulk1−/− HbbTh3/+ reticulocytes. Thus, we conclude that Ulk1 mediates the degradation of free α-globin in β-thalassemia.
Our findings illustrate a new mechanism through which protein quality-control pathways can modulate the severity of β-thalassemia by eliminating unstable free α-globin, and they open up possibilities for new therapeutic approaches, as Ulk1 activity can be modulated pharmacologically.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.